IL-6 Signaling Pathway
Interleukin-6 (IL-6) is a pleiotropic cytokine involved in various physiological and pathological processes. It’s signaling pathway plays a crucial role in immune responses, inflammation, and cellular homeostasis [2]. Understanding the intricate mechanisms underlying IL-6 signaling is essential for developing targeted therapeutic interventions for numerous diseases. OriGene has developed products to help you study the IL-6 pathway efficiently. Look at our portfolio.
Classical and Trans IL-6 Signaling Pathway
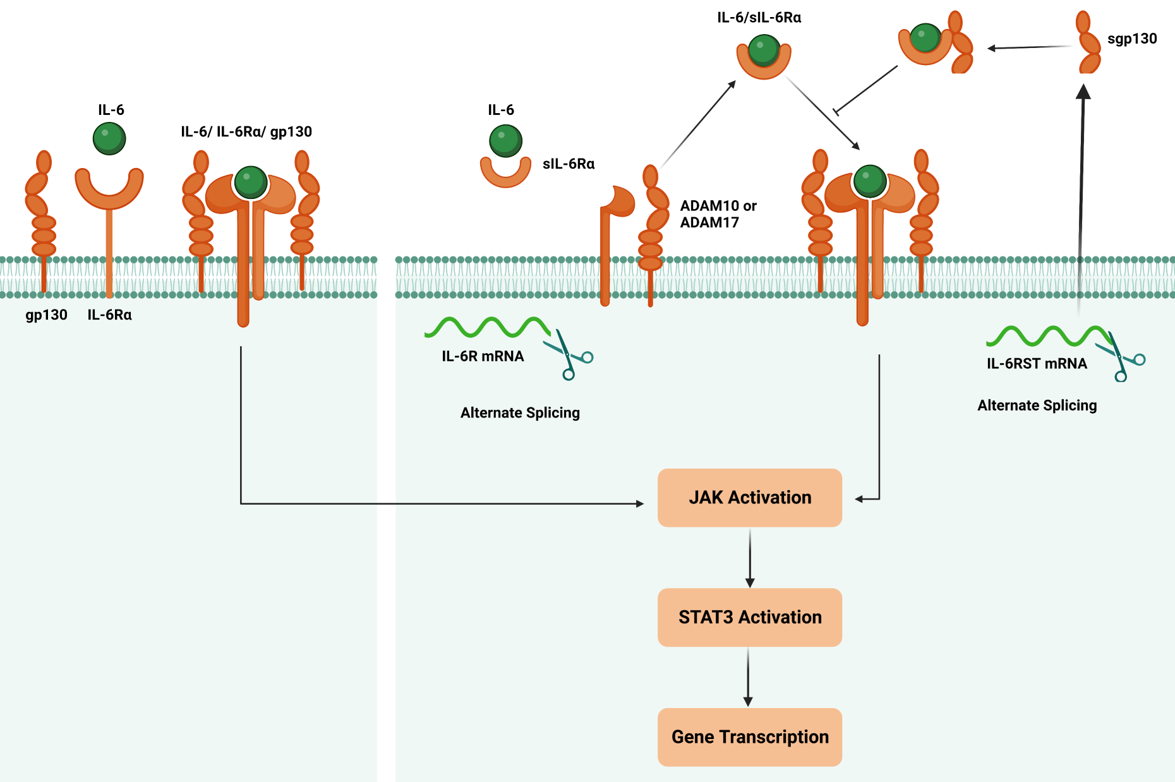
The IL-6 cytokine family has several members that bind to the gp130 receptor subunit to initiate intracellular signaling. There are two IL-6 Signaling Pathways i) Classical Signaling ii) Trans Signaling Pathway. In both pathways, IL-6 elicits its effects by engaging its receptor complex, composed of IL-6Rα and gp130. Upon ligand binding, IL-6 receptor activation leads to a cascade of events that modulate gene expression and trigger diverse cellular responses [3].
a)IL-6 Classical Pathway: IL-6 receptor activation begins with the binding of IL-6 to IL-6Rα, inducing a conformational change in the receptor complex. This facilitates the recruitment and dimerization of gp130, leading to the formation of a hexameric signaling complex. The hexamerization of gp130 initiates intracellular signaling by promoting phosphorylation events and downstream signaling cascades, including the JAK/STAT, MAPK/ERK, and PI3K/AKT pathways [3]. These pathways are essential in mediating IL-6-induced gene expression, cellular proliferation, differentiation, survival, and immune responses.
b)IL-6 Trans Signaling Pathway: Involves the soluble form of the IL-6 receptor (sIL-6R) and the glycoprotein 130 (gp130) receptor. This pathway allows IL-6 to exert its biological effects on cells that lack the membrane-bound IL-6 receptor (mIL-6R). The IL-6 trans-signaling pathway is initiated by forming a complex between IL-6 and sIL-6R in the extracellular space. This complex can diffuse away from its site of origin, allowing IL-6 to reach cells that do not express the mIL-6R. Upon encountering such cells, the IL-6/sIL-6R complex binds to gp130, ubiquitously expressed on these cells' surfaces. The binding of the IL-6/sIL-6R complex to gp130 induces conformational changes that activate JAKs associated with the cytoplasmic domain of gp130. Activated JAKs phosphorylate specific tyrosine residues on gp130, providing docking sites for downstream signaling molecules.
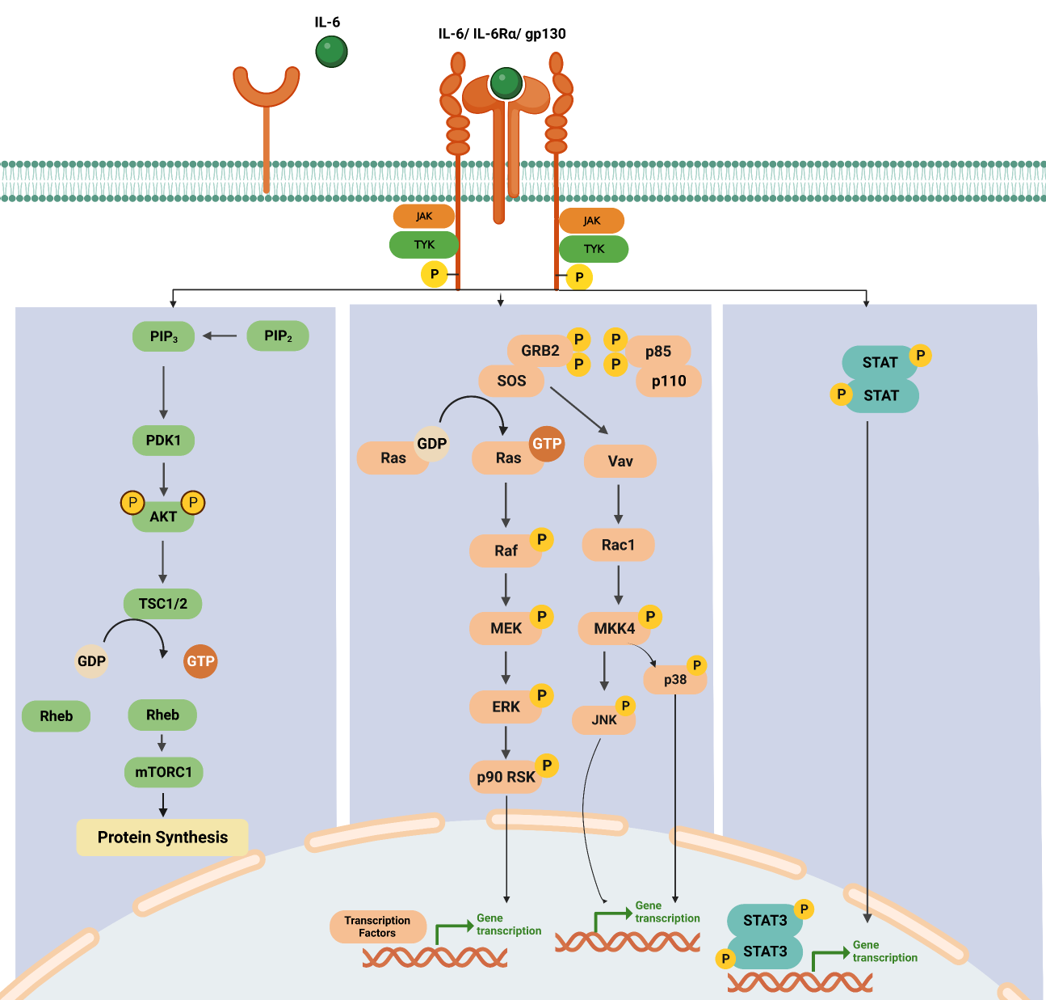
Downstream Signaling of the IL-6 Receptor
IL-6 binds to the IL-6 receptor (IL-6R), which consists of two subunits: IL-6Rα and gp130. IL-6 binding induces the dimerization of two IL-6Rα subunits and the recruitment of two gp130 subunits, forming a hexameric receptor complex. The hexameric receptor complex activates JAK family members, particularly JAK1 and JAK2, associated with the cytoplasmic domain gp130. JAK1 and JAK2 phosphorylate each other and specific tyrosine residues on the cytoplasmic domain of gp130. This phosphorylation creates docking sites for downstream signaling molecules.
In the STAT pathway, Phosphorylated gp130 acts as a docking site for STAT proteins, particularly STAT3.STAT3 proteins are recruited to the phosphorylated gp130, JAK1, and JAK2 phosphorylate. Once phosphorylated, STAT3 forms homodimers/heterodimers with the same or different isoforms of STAT and translocates from the cytoplasm into the nucleus. In the nucleus, STAT3 dimers bind to specific DNA sequences, known as STAT response elements (SREs), in the promoter regions of target genes, binding of STAT3 to SREs leads to the transcriptional activation of target genes, including those involved in immune responses, cell proliferation, survival, and inflammation [4].
The formation of either the IL-6 classic or trans-signaling ligand-receptor complexes initiates the activation of several intracellular signaling pathways, including the Jak-STAT pathway, the Ras-MAPK pathway, the p38 and JNK MAPK pathways, the PI3K-Akt pathway, and the MEK-ERK5 pathway. While both the classic and trans-signaling receptor complexes activate similar intracellular signaling pathways, emerging evidence suggests that classic signaling is essential for the anti-inflammatory and regenerative effects of IL-6, whereas IL-6 trans-signaling tends to promote pro-inflammatory responses.
Reference
- Johnson, Daniel E., Rachel A. O'Keefe, and Jennifer R. Grandis. "Targeting the IL-6/JAK/STAT3 signalling axis in cancer. "Nature reviews Clinical oncology 15.4 (2018): 234-248.
- Tanaka, Toshio, Masashi Narazaki, and Tadamitsu Kishimoto. "IL-6 in inflammation, immunity, and disease. "Cold Spring Harbor perspectives in biology 6.10 (2014): a016295.
- Rose-John, Stefan. "IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. "International journal of biological sciences 8.9 (2012): 1237.
- Morris, Rhiannon, Nadia J. Kershaw, and Jeffrey J. Babon. "The molecular details of cytokine signaling via the JAK/STAT pathway. "Protein Science 27.12 (2018): 1984-2009.


